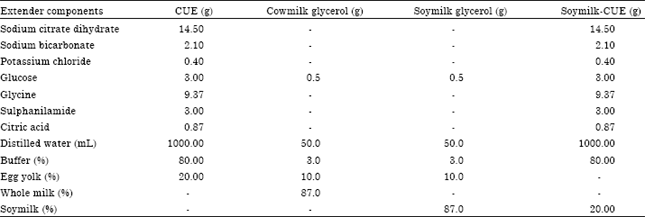
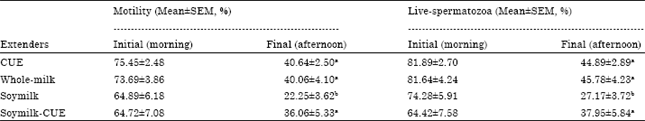
Research Article
Effects of Extender Types on Ram Semen Collected with Electroejaculator in a Tropical Environment
Reproductive Physiology Unit, Department of Animal Production, Faculty of Agriculture, University of Ilorin, P.M.B. 1515, Ilorin, Kwara State, Nigeria
A.A. Adeloye
Reproductive Physiology Unit, Department of Animal Production, Faculty of Agriculture, University of Ilorin, P.M.B. 1515, Ilorin, Kwara State, Nigeria
K.D. Adeyemi
Reproductive Physiology Unit, Department of Animal Production, Faculty of Agriculture, University of Ilorin, P.M.B. 1515, Ilorin, Kwara State, Nigeria
O.A. Olatunde
Reproductive Physiology Unit, Department of Animal Production, Faculty of Agriculture, University of Ilorin, P.M.B. 1515, Ilorin, Kwara State, Nigeria
V. Ojo
Reproductive Physiology Unit, Department of Animal Production, Faculty of Agriculture, University of Ilorin, P.M.B. 1515, Ilorin, Kwara State, Nigeria
F.E. Sola-Ojo
Reproductive Physiology Unit, Department of Animal Production, Faculty of Agriculture, University of Ilorin, P.M.B. 1515, Ilorin, Kwara State, Nigeria