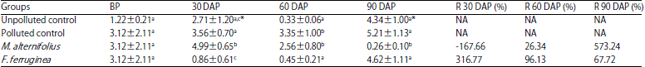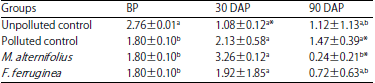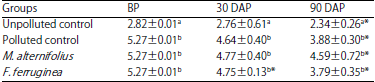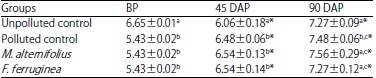Research Article
Enzymatic Recovery of Crude Oil Polluted Soil Enhanced by Treatment using Mariscus alternifolius Vahl. and Fimbristylis ferruginea
Department of Biochemistry, Faculty of Science, University of Port Harcourt, P.M.B. 5239, Choba, Rivers State, Nigeria
LiveDNA: 234.25603
Jude Chigozie Ikewuchi
Department of Biochemistry, Faculty of Science, University of Port Harcourt, P.M.B. 5239, Choba, Rivers State, Nigeria
Michael Okechukwu Monanu
Department of Biochemistry, Faculty of Science, University of Port Harcourt, P.M.B. 5239, Choba, Rivers State, Nigeria