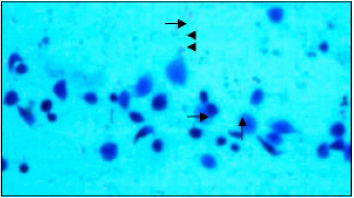
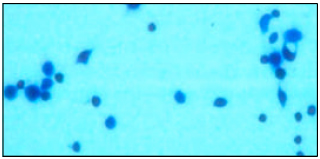
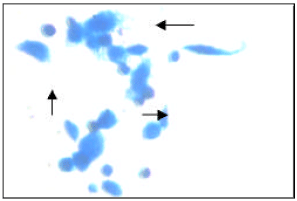
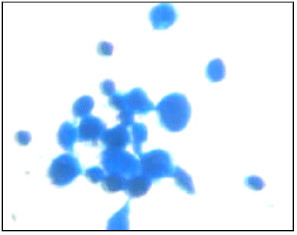
Research Article
Effect of Temperature on Hemocytic Immune Responses of Tropical Tasar Silkworm, Antheraea mylitta D
Laboratory of Silkworm Physiology, Central Tasar Research and Training Institute, Central Silk Board, P.O. Piska-Nagri, Ranchi-835003, India
P.K. Mishra
Laboratory of Silkworm Physiology, Central Tasar Research and Training Institute, Central Silk Board, P.O. Piska-Nagri, Ranchi-835003, India
D. Kumar
Laboratory of Silkworm Physiology, Central Tasar Research and Training Institute, Central Silk Board, P.O. Piska-Nagri, Ranchi-835003, India
B.M.K. Singh
Laboratory of Silkworm Physiology, Central Tasar Research and Training Institute, Central Silk Board, P.O. Piska-Nagri, Ranchi-835003, India
B.C. Prasad
Laboratory of Silkworm Physiology, Central Tasar Research and Training Institute, Central Silk Board, P.O. Piska-Nagri, Ranchi-835003, India