Research Article
Adsorption of Heavy Metals from Aqueous Solutions on Synthetic Zeolite
Central Metallurgical R and D Institute (CMRDI), P.O. Box 87, Helwan 11421, Cairo, Egypt
Several industrial wastewater streams may contain heavy metals such as Cr, Cu, Pb, Zn, Co, Ni, Mn, etc. including the waste liquids generated by metal finishing or the mineral processing industries. Heavy metals are considered hazardous pollutants due to their toxicity, even at low concentration and nonbiodegradability. Increasing level of heavy metals in natural water bodies poses a serious threat to all living species including humans. It is, therefore, essential to reduce the heavy metal concentration in effluents/wastewater before it is discharged into the water bodies (Kurniawan et al., 2006).
The most common methods available to reduce heavy metal concentration are chemical precipitation, ion-exchange, adsorption and reverse osmosis. Most of these methods suffer from some drawbacks such as high capital and operational costs and problem of disposal of residual metal sludge (Kurniawan et al., 2006; Hui et al., 2005). This has encouraged research into using low-cost adsorbent materials to purify water contaminated with metals. Among these methods, adsorption and ion exchange using natural, synthetic and modified inorganic and organic solids have been explored (Bailey et al., 1999; Blais et al., 2003; Dal Bosco et al., 2005; Al-Qunaibit et al., 2005; Prado et al., 2005; Wingenfelder et al., 2005). In this group, clay minerals and zeolite act as potential ionic exchangers for heavy metals due to their low cost, high abundance, easy manipulation and harmlessness to the environment (Ghobarkar et al., 1999; da Fonseca et al., 2006). Crystalline structures of most clay minerals are generated by a combination of octahedral and tetrahedral sheets normally classified in two groups of hydrous phyllosilicates that have the inorganic structural arrangement in 1:1 or 2:1 layers. Zeolites are hydrated aluminosilicates of alkali and alkaline earth elements with unique crystal structures consisting of a three-dimensional framework of SiO4 and AlO4 tetrahedral. This structure causes zeolite to have negatively charged surface which can be used for adsorption of metals (Ghobarkar et al., 1999; Kaya and Durukan, 2004).
Several researchers have studied the removal performance and selectivity sequence of heavy metal ions by natural zeolites (Ouki and Kavannagh, 1997; Singh et al., 2000; Inglezakis et al., 2002, 2003; Panayotova and Velikov, 2003) as well as synthetic zeolites (Moreno et al., 2001; Querol et al., 2002; Alvarez-Ayuso et al., 2003). Ouki and Kavannagh (1997) studied the performance of natural zeolites (clinoptilolite and chabazite) on the treatment of mixed metal effluents {Pb(II), Cd(II), Cu(II), Zn(II), Cr(III) and Co(II)}, the adsorption of such metals was found to be a pseudo-first-order kinetic reaction (Panayotova and Velikov, 2003). Alvarez-Ayuso et al. (2003) studied the sorption behavior of Cr(III), Ni(II), Zn(II), Cu(II) and Cd(II) ions by natural (clinoptilolite) and synthetic (NaP1) zeolites. They found that the sorption capacity of synthetic NaP1 zeolite was 10 times greater than the natural zeolite. Babel and Kurniawan (2003) studied Cr(VI) uptake from simulated wastewater using natural zeolite. NaCl treated zeolite had better removal capabilities (3.23 mg g-1) for Cr(VI) ions than as-received zeolite (1.79 mg g-1) at an initial Cr concentration of 20 mg L-1. These results suggest that the Cr(VI) adsorption capacities by zeolite varied, depending on the extent of chemical treatment (Wingenfelder et al., 2005). The results were significantly lower than those of Peric' et al. (2004), who also used zeolite for Zn(II) and Cu(II) removal. Zamzow and Murphy (1992) and Later, Colella et al. (1998) investigated the removal capacity of a wide variety of zeolite minerals for cadmium, copper and zinc. They revealed that zinc had the lowest adsorbed ions, among others, by all types of zeolites. Trgo and Peric (2003) recommended the use of zeolite tuff as an ion exchanger in the technological processes of water with low Zn ion concentrations. Oren and Kaya (2006) investigated the efficiency of two natural zeolites from Turkey, which consist mainly of clinoptilolite, in the removal of zinc ions from aqueous solutions. The adsorption behavior of both zeolites was highly dependent on the pH and the initial zinc ion concentration. The adsorption capacity of two commercial zeolites (zeolite X and clinoptilolite) had been studied by performing batch tests in Zn(II) aqueous solutions at neutral pH (Veronica et al., 2003). The results showed that zeolite surface reactivity was greatly influenced by the mineral cage-like structure and particularly the presence of pockets, spaces and channels.
The aim of this study is to study the adsorption behavior of heavy metal ions specifically, Cu(II), Zn(II), Mn(II) and Cr(VI) from aqueous solution on synthetic 4A Zeolite. Different parameters affecting the adsorption processes such as pH, contact time, zeolite and initial metal ions concentration were investigated.
Adsorbate
All chemicals used to prepare the reagent solutions were of technically analytical grade. Heavy metal ion solutions of certain concentrations each of Cu(II), Zn(II) and Cr(VI) were prepared by dissolving weighed quantities of copper(II) chloride, zinc(II) chloride, potassium dichromate salts in bidistilled water. Manganese dioxide was dissolved in 1M HCl for preparation of Mn(II) solution.
Adsorbent
Synthetic 4A zeolite was provided by the Evaluation Laboratory of CMRDI, Egypt. The 4A zeolite was synthesized previously by calcining of law grade Kalabsha Kaolin for 2 h in air at 800°C and reacted hydrothermally with 2-4 M NaOH solution at 100°C for 2 h. The obtained gel was filtered, washed till pH 10, the product was agitated for 1 h at 95°C and dried at 100°C for 24 h. The produced 4A zeolite is characterized by high crystallinity with brightness range of 78.8-82.2 and average particle size of 5 μm (Hassan et al., 2002).
Batch Adsorption Experiments
Adsorption of different heavy metal ions {Cu(II), Zn(II), Mn(II) and Cr(VI)} was carried out in 100 mL stoppered conical flask by taking appropriate amounts of metal ion solution and adsorbent.
The pH values of the solutions were adjusted from 1 to 11 with 0.5 M NaOH and 0.5 M HClO4 and measured with an Orion Model 801A pH meter. The pH was kept at a specific value during experiments using a pH controller (New Brunswick Scientific, Edison, NJ). The experiments were carried out for 120 min. The liquid samples were filtered after adsorption for metal ions analysis by Atomic Absorption Spectroscopy (AAS).
Adsorption Capacity
The adsorbents (0.25 g) were left in contact with 100 mL of each of heavy metal ions solutions in the range of 50-1000 mg L-1 with the initial pH value of 9 for 60 min. The filtrates were filtered for metal ions analysis by AAS. The amount of metal ions adsorption onto the zeolite can be calculated (Oren and Kaya, 2006) by:
| (1) |
where, qe is the metal ions adsorbed onto the zeolite (mg g-1), Ci the initial metal ion concentration (mg L-1), Ce the final metal ion concentration in the solution (mg L-1) and S is the slurry concentration (g L-1).
Effect of 4A Zeolite Grain Size
Table 1 show the grain size distribution of the 4A zeolite sample. It is clear that the fraction F1 with particle size of >90-200 μm, represents 50% of the sample.
Figure 1 shows the effect of the particle size of the sample on the adsorption of the studied metal ions. It can be seen that the grain size has a little influence on the adsorption capacity of the metal ions. The three fractions of the zeolite sample showed a high adsorption capacity. However the less fine fraction F3 with grain size of >300 μm showed a relatively lower adsorption capacity. This may be attributed to the relatively lower surface area of F3 fraction.
| Table 1: | The particle size distribution of the 4A zeolite sample |
 | |
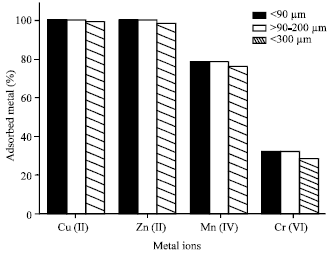 | |
| Fig. 1: | Effect of grain size fractions of 4A zeolite on the metal ions adsorption |
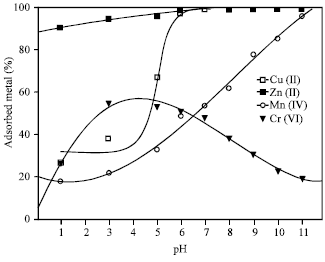 | |
| Fig. 2: | Effect of pH on the adsorption of the heavy metal ions on 4A zeolite Mn+ = 100 mg L-1, t = 1 h, zeolite concentration = 2.5 g L-1 |
Effect of pH
After determining the effect of grain size of the zeolite on the metal ions adsorption, the two fractions, F1 and F2, of grain size <90 μm and >90-200 μm were mixed and chosen for the batch adsorption experiments. The solution pH plays a major role in determining the amount of metal ions adsorbed. Adsorption was studied over the range of pH up to 11 and the results are shown in Fig. 2. The initial metal ion concentrations were kept constant at 100 mg L-1. Adsorption of Cu(II) and Zn(II) ions increased rapidly with increase of pH, a complete adsorption for the metal ions were achieved at pH >6. The rapid increase in the adsorption is partly attributed to the formation of different hydroxo species with rise in solution pH. Based on the hydrolysis constants (Martell and Smith, 1977; Horsfall and Abia, 2003) of different metal ions as defined in and taking only primary metal species expected to be formed in the working pH range into consideration,
| (2) |
It is evident that dihydroxo species are the predominating to a significant extent above pH>6 for Cu(II) and Zn(II) (Das and Jana, 2006). Since maximum adsorption for Cu(II) and Zn(II) was achieved at pH >6, it may safely be stated that the removal of Cu(II) and Zn(II) was mostly due to adsorption and not precipitation. However, precipitation of small fractions of Cu(II) and Zn(II) even at pH>6 on the surface by nucleation cannot be neglected. At still higher pH (>6), however, part of Cu(II) and Zn(II) may be precipitated as dihydroxo species, which also depend upon the initial metal ion concentration. Adsorption of Mn(II) ions was gradually increased with increase of pH reaching maximum adsorption capacity of 96% at pH 11.
On the other hand, the adsorption of Cr(VI) was achieved at acidic pH values reaching a maximum adsorption of about 55% at pH range of 3-5. A further increase in the pH resulted in decreasing the adsorption capacity of Cr(VI) ions. At the low pH, the dominant species of Cr(VI) in solution are anionic species like HCrO4¯ and Cr2O72¯ (Alvarez et al., 2006). Chemisorption of anions on variable-charge minerals can be affected by pH in two different ways, the speciation of solute species and the surface charge of mineral. low pH values favour the protonization of hydroxyl groups and so their replacement by oxyanions by means of the ligand exchange mechanism, since water molecules are easier to displace from metal binding sites than hydroxyl groups (Alvarez-Ayuso et al., 2006). This could be in agreement with the hypothesis that Cr(VI) is adsorbed by means of an ion-exchange mechanism (Yua et al., 2003).
Effect of Time
Figure 3 shows the effect of contact time of 2 h, at pH 9, on the adsorption capacity of the metal ions. The adsorption increases with increasing in the contact time reaching maxima adsorption values of 99.9, 99.9, 81.8 and 37.5% for Cu(II), Zn(II), Mn(II) and Cr(VI), respectively. The uptake of both Cu(II) and Zn(II) was very fast in the first 10 min, while Mn(II) and Cr(VI) were gradually adsorbed. The exchange equilibrium was established with in 1 h for all the metal ions.
Effect of Slurry Concentration
Figure 4 shows the effect of zeolite slurry concentration on the adsorption capacity of the metal ions. The other parameters (such as contact time of 1 h, pH 9 and initial metal concentration of 100 mg L-1) were kept constant. The adsorption increases with increasing in the zeolite concentration. The uptake of both Cu(II) and Zn(II) was very high with 1 g L-1 zeolite compared to that occurred with Mn(II) and Cr(VI). The exchange equilibrium was established with zeolite concentration of 2.5 g L-1 for all the metal ions.
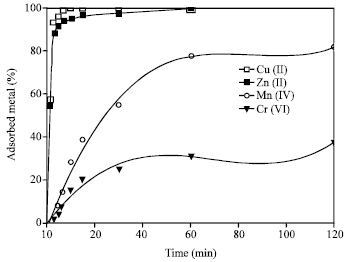 | |
| Fig. 3: | Effect of time on the adsorption of the heavy metal ions on 4A zeolite Mn+ = 100 mg L-1, zeolite concentration = 2.5 g L-1, pH = 9 |
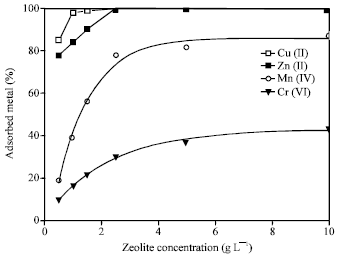 | |
| Fig. 4: | Change in adsorption of heavy metal ions on various concentrations of 4A zeolite Mn+ = 100 mg L-1, t = 1 h, pH = 9 |
Effect of Initial Metal Concentration
The adsorbed ions were highly dependent on the initial metal ion concentration (Fig. 5). Sorption of the metal ion decreased with increasing the initial metal ion concentration. A significant decrease in the sorption of both Mn(II) and Cr(VI) was achieved with increasing their initial concentration. However, almost all the Cu(II) and most of Zn(II) were adsorbed event at high initial concentration. This may be attributed to the higher selectivity of zeolite towards Cu(II) and Zn(II) ions. The selectivity behavior could be the result of various factors, such as framework structure of zeolite, hydrated size and hydration free energy of metal ions (Hui et al., 2005). Another mechanism for the high sorption of both Cu(II) and Zn(II) ions, is based on the possibility of precipitation of metal hydroxide on the surface of zeolite or inside the pore walls at the alkaline pH value.
Adsorption Isotherms
Adsorption isotherm can be used to describe how solutes interact with adsorbent and so is critical in optimizing the use of adsorbent. The sorption of the metal ions was carried out at different initial metal ion concentrations ranging from 50 to 1000 mg L-1, with the optimum agitation period (1 h) at the optimum pH value for each metal ion {pH 9 for both of Cu(II) and Zn(II), pH 11 for Mn(II) and pH 3 for Cr(VI)}. The adsorption capacity decreased in the order Cu(II) > Zn(II) > Mn(II) >Cr(VI) (Fig. 6). The different adsorption affinity of the zeolite towards the metal ions can be explained as follows; Zeolites, in general, are weakly acidic in nature and therefore sodium form exchangers are selective for hydrogen (R-Na + H2O > RH + Na+ + OH¯). This leads to high pH values when the exchanger is equilibrated with a relatively dilute electrolyte solution (Leinonen and Lehto, 2001), making feasible the metal hydroxide precipitation. The crystal structure of 4A zeolite contains large cages having a near spherical shape and free diameter of 11.4 A°. The effective pore width of zeolite 4A is 4 A°. A common factor preventing a group of metal ions from being adsorbed by zeolite 4A is the size of the hydrated ion. If the hydrated ion size is greater than that of the pore, the species may be excluded or some of the waters of hydration must be stripped from the solvated ions to enable them to enter the pores of the zeolite. Also, the metal with the highest free energy of hydration should therefore prefer to remain in the solution phase (Hui et al., 2005).
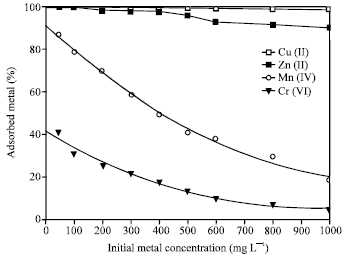 | |
| Fig. 5: | Change in adsorption of heavy metal ions on 4A zeolite with the initial metal ions concentrations zeolite concentration = 2.5 g L-1, t = 1 h, pH = 9 |
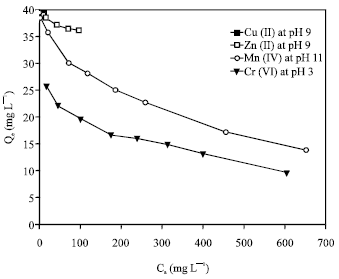 | |
| Fig. 6: | Adsorption isotherms for removal of the heavy metal ions by 4A zeolite at the optimum solution pH values |
Two kinds of several isotherm equations have been applied for this study, Langmuir and Freundlich isotherms. The two isotherm models are the simplest and most commonly used isotherms to represent the adsorption of components from a liquid phase onto a solid phase (Hui et al., 2005; Das and Jana, 2006; Baran et al., 2006). The Langmuir model assumes monolayer adsorption, while the Freundlich model is empirical in nature (Boddu et al., 2003). The Langmuir sorption isotherm is represented as:
| (3) |
where, Ce is the equilibrium aqueous metal ions concentration (mg L-1), qe the amount of metal ions adsorbed per gram of adsorbent at equilibrium (mg g-1), qm and b are the Langmuir constants related to the maximum adsorption capacity and energy of adsorption, respectively. The values of qm (mg L-1) and b (mg-1) can be determined from the linear plot of Ce/qe versus Ce.
The Freundlich isotherm can be used for a non-ideal sorption that involves heterogeneous sorption and is expressed as:
| (4) |
where, K is roughly an indicator of the adsorption capacity and 1/n the adsorption intensity. By plotting log qe versus logCe, values of K and n can be determined from the slope and intercept of the plot.
The equilibrium adsorption data obtained were fitted to linearly transformed Langmuir and Freundlich equations as shown in Fig. 7 and 8, respectively. It can be seen that for the all studied metal ions, the Langmuir model represents a better fit to the experimental data than the Freundlich model. The good agreement of the Langmuir plots with the experimental data suggests a monolayer coverage of the metal ions on the outer surface of the adsorbent.
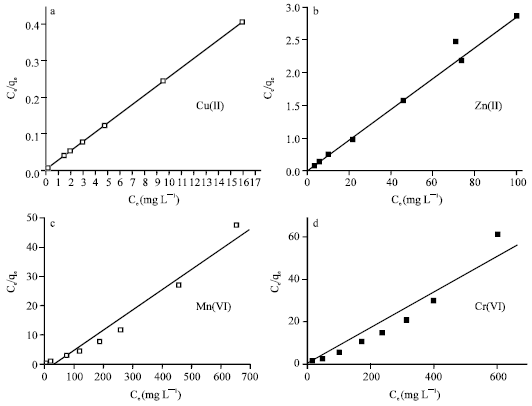 | |
| Fig. 7: | Linearized Langmuir isotherms for removal of the heavy metal ions by 4A zeolite |
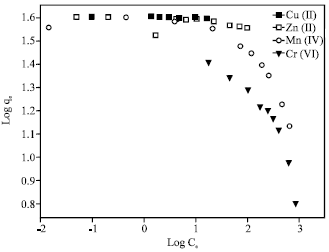 | |
| Fig. 8: | Freundlich isotherms for removal of the heavy metal ions by 4A zeolite |
The adsorption capacity of 4A synthetic zeolite was evaluated for uptake of Cu(II), Zn(II), Mn(II) and Cr(VI) from aqueous solution. The grain size of the 4A zeolite had a little effect on the adsorption capacity, the tested zeolite sample was of grain size <90-200 μm. The adsorption capacity of the metal ions were found to be strongly dependent on pH. Complete adsorption for Cu(II) and Zn(II) ions was achieved at pH >6, while the adsorption capacity for both Mn(II) and Cr(VI) was 96 and 55.3% at pH 11 and pH 3, respectively. The optimum contact time was 1 h with initial metal ion concentration of 100 mg L-1. The exchange equilibrium was established with zeolite concentration of 2.5 g L-1 for all the metal ions. The removal mechanism of the metal ions was mainly by adsorption and ion exchange processes. The experimental data showed a good agreement of the Langmuir plots which suggest a monolayer coverage of the metal ions on the outer surface of the adsorbent.