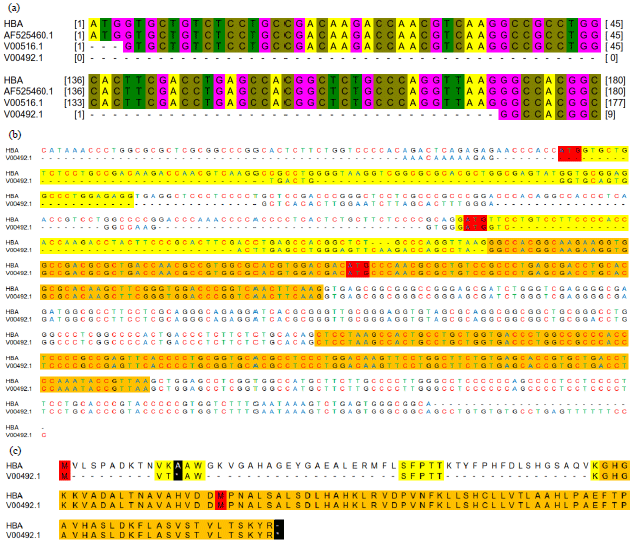
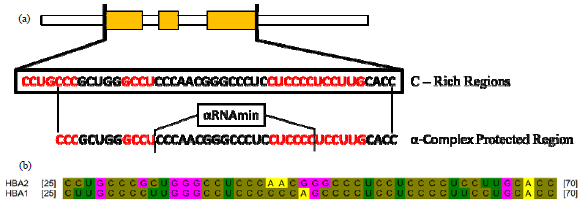
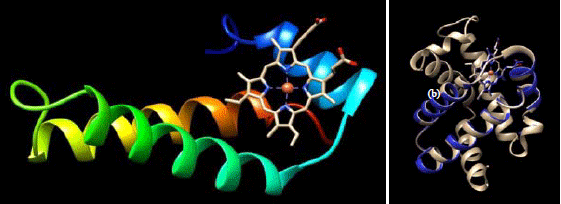
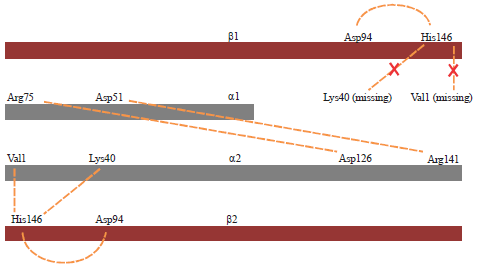
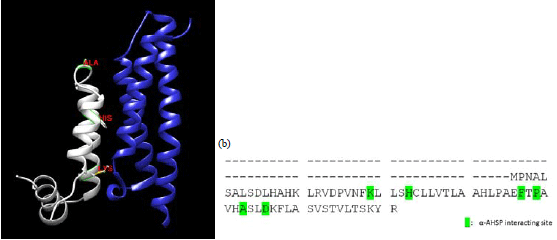
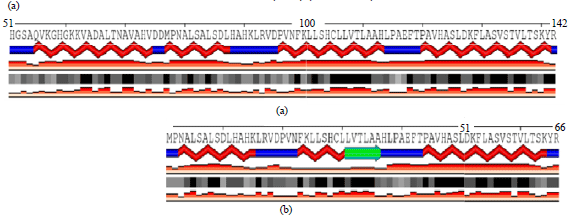
Research Article
α-globin Alteration in α-thalassemia Disorder: Prediction and Interaction Defect
Laboratory of Genetics and Breeding, Faculty of Biology, Universitas Gadjah Mada, Jl. Teknika Selatan, Sekip Utara, Yogyakarta 55281, Indonesia Tel/Fax: +62-274-580839
LiveDNA: 62.16385
Nailil Husna
Laboratory of Genetics and Breeding, Faculty of Biology, Universitas Gadjah Mada, Jl. Teknika Selatan, Sekip Utara, Yogyakarta 55281, Indonesia Tel/Fax: +62-274-580839
Immanuel Sanka
Laboratory of Genetics and Breeding, Faculty of Biology, Universitas Gadjah Mada, Jl. Teknika Selatan, Sekip Utara, Yogyakarta 55281, Indonesia Tel/Fax: +62-274-580839