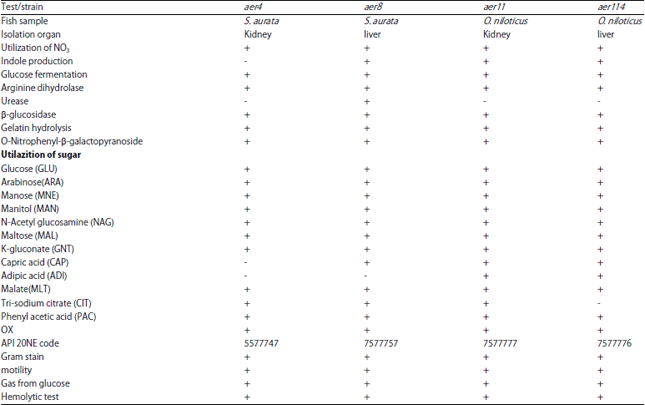
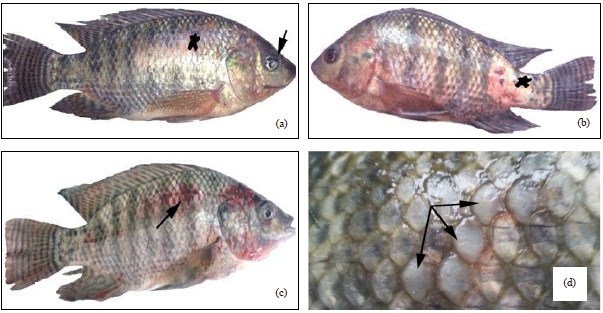
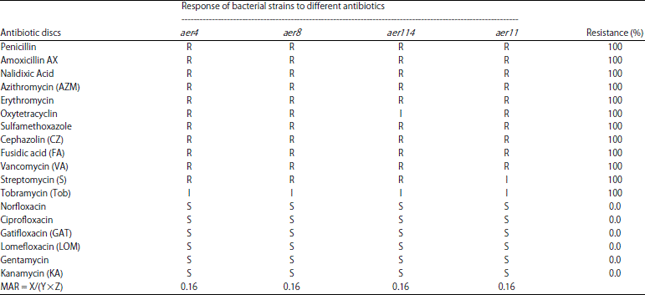
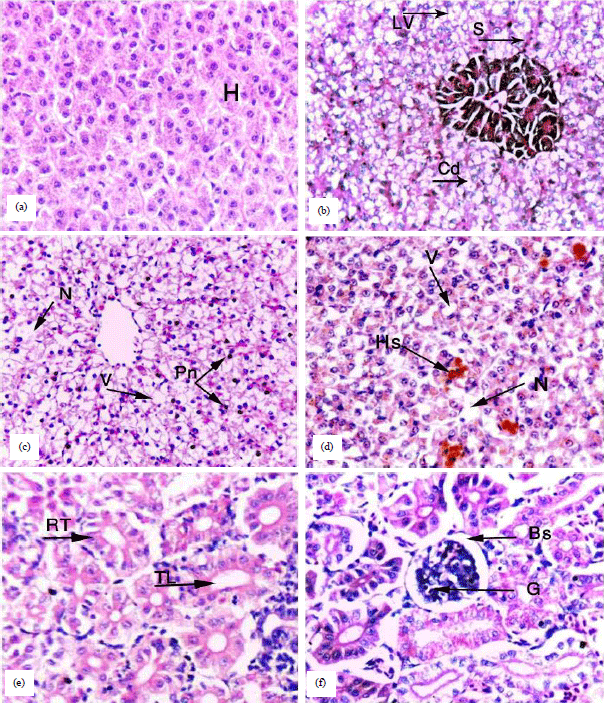
Research Article
Serum Biochemical and Histopathological Changes Associated with Aeromonas hydrophila Isolated from Oreochromis niloticus and Sparus aurata with Multiple Antibiotic Resistance Index
Laboratory of Fish Disease, National Institute of Oceanography and Fisheries, Alexandria, Egypt
LiveDNA: 20.15073