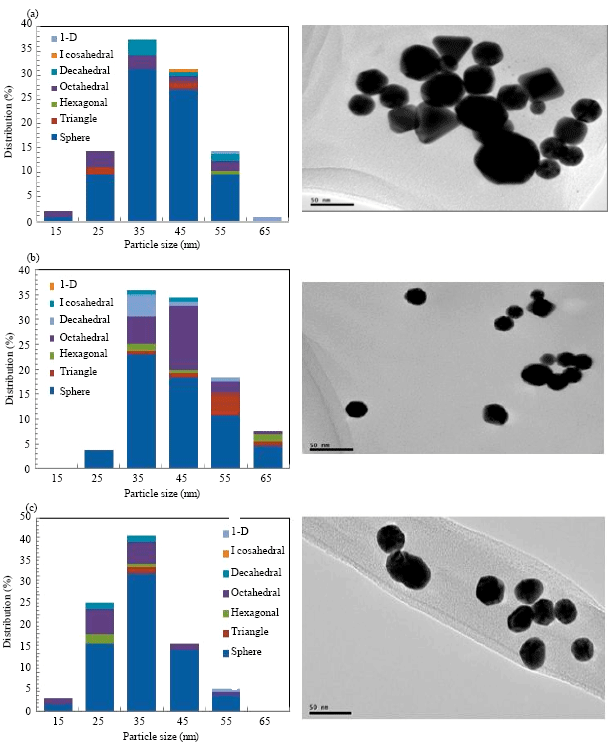
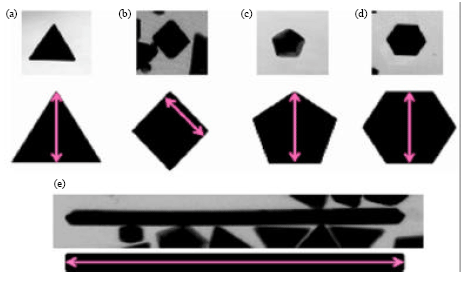
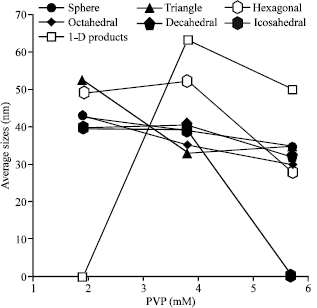
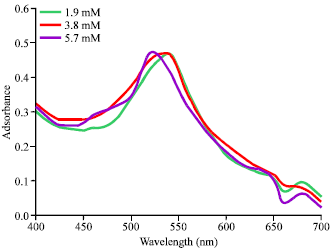
Research Article
Effects of PVP Concentration on the Formation of Size and Shape of Gold (Au) Nanoparticles for Mercury Adsorption
Department of Gas Engineering, Faculty of Chemical and Natural Resources Engineering, Universiti Teknologi Malaysia, 81310 UTM Skudai, Johor, Malaysia
K.S.N. Kamarudin
Department of Gas Engineering, Faculty of Chemical and Natural Resources Engineering, Universiti Teknologi Malaysia, 81310 UTM Skudai, Johor, Malaysia
N.N.F. Nik Mohamed Fathilah
Department of Gas Engineering, Faculty of Chemical and Natural Resources Engineering, Universiti Teknologi Malaysia, 81310 UTM Skudai, Johor, Malaysia
M.S. Mohamed
Ibnu Sina Institute for Fundamental Science Studies, Universiti Teknologi Malaysia, 81310 UTM Skudai, Johor, Malaysia