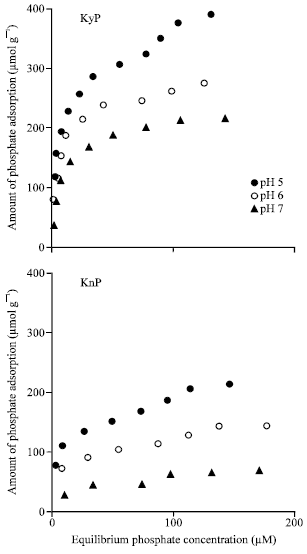
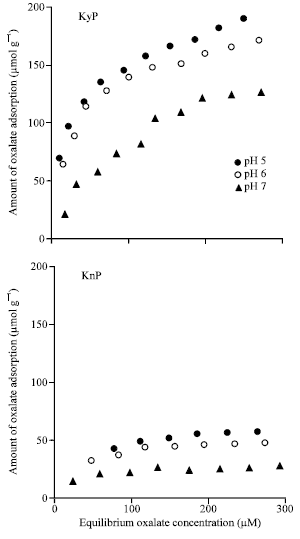
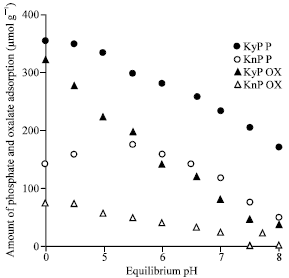
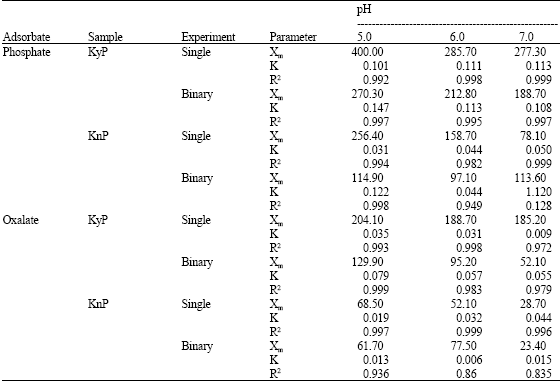
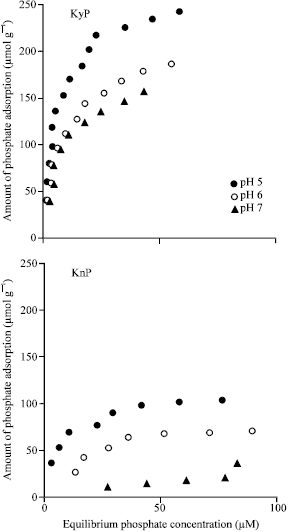
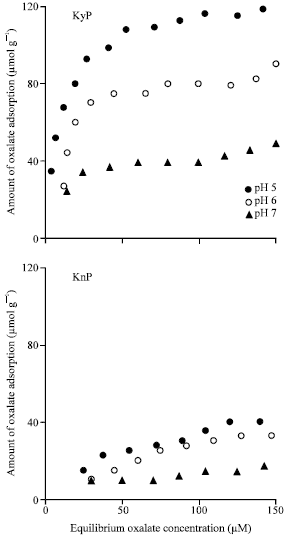
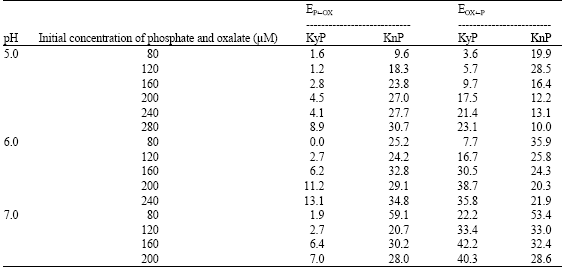
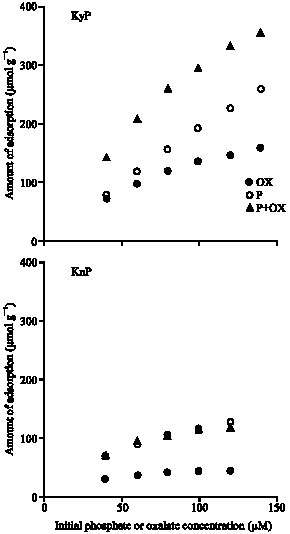
Research Article
Effect of Si/Al Ratio of Allophane on Competitive Adsorption of Phosphate and Oxalate
Laboratory of Applied Chemistry for Environment Industry, Faculty of Agriculture, Ehime University, 3-5-7 Tarumi, Matsuyama 790-8566, Japan
N. Matsue
Laboratory of Applied Chemistry for Environment Industry, Faculty of Agriculture, Ehime University, 3-5-7 Tarumi, Matsuyama 790-8566, Japan
T. Henmi
Laboratory of Applied Chemistry for Environment Industry, Faculty of Agriculture, Ehime University, 3-5-7 Tarumi, Matsuyama 790-8566, Japan